Divalent atom functions#
Note
This is a completely new module added in ARC 3.0.0 version. See more at E. J. Robertson, N. Šibalić, R. M. Potvliege and M. P. A. Jones, *Computer Physics Communications* **261**, 107814 (2021) `https://doi.org/10.1016/j.cpc.2020.107814 .
Overview#
DivalentAtom Methods
|
Dipole matrix element \(\langle n_1 l_1 j_1 m_{j_1} |e\mathbf{r}|\ n_2 l_2 j_2 m_{j_2}\rangle\) in units of \(a_0 e\) |
|
Calculated transition wavelength (in vacuum) in m. |
|
Calculated transition frequency in Hz |
|
Returns a Rabi frequency for resonantly driven atom in a center of TEM00 mode of a driving field |
|
Returns a Rabi frequency for resonant excitation with a given electric field amplitude |
|
Returns the lifetime of the state (in s) |
|
Transition rate due to coupling to vacuum modes (black body included) |
Reduced matrix element in \(J\) basis, defined in asymmetric notation. |
|
Reduced matrix element in \(J\) basis (symmetric notation) |
|
Reduced matrix element in \(L\) basis (symmetric notation) |
|
|
Radial part of the dipole matrix element |
Radial part of the quadrupole matrix element |
|
|
Vapour pressure (in Pa) at given temperature |
|
Atom number density at given temperature |
Returns average interatomic spacing in atomic vapour |
|
|
Energy of the level relative to the ionisation level (in eV) |
|
Retuns linear (paramagnetic) Zeeman shift. |
|
Quantum defect of the level. |
|
C6 interaction term for the given two pair-states |
|
C3 interaction term for the given two pair-states |
|
Energy defect for the given two pair-states (one of the state has two atoms in the same state) |
|
Energy defect for the given two pair-states |
Updates the file with pre-calculated dipole matrix elements. |
|
|
Returns radial part of the coupling between two states (dipole and quadrupole interactions only) |
|
Average (mean) speed at a given temperature |
|
Returns literature information on requested transition. |
Detailed documentation#
- class DivalentAtom(preferQuantumDefects=True, cpp_numerov=True)[source]#
Bases:
arc.alkali_atom_functions.AlkaliAtomImplements general calculations for Alkaline Earths, and other divalent atoms.
This class inherits
arc.alkali_atom_functions.AlkaliAtom. Most of the methods can be directly used from there, and the source for them is provided in the base class. Few methods that are implemented differently for Alkaline Earths are defined here.- Parameters
preferQuantumDefects (bool) – Use quantum defects for energy level calculations. If False, uses NIST ASD values where available. If True, uses quantum defects for energy calculations for principal quantum numbers within the range specified in
defectFittingRangewhich is specified for each element and series separately. For principal quantum numbers below this value, NIST ASD values are used if existing, since quantum defects. Default is True.cpp_numerov (bool) – This switch for Alkaline Earths at the moment doesn’t have any effect since wavefunction calculation function is not implemented (d.m.e. and quadrupole matrix elements are calculated directly semiclassically)
- breitRabi(n, l, j, B)#
Returns exact Zeeman energies math:E_z for states \(\vert F,m_f\rangle\) in the \(\ell,j\) manifold via exact diagonalisation of the Zeeman interaction \(\mathcal{H}_z\) and the hyperfine interaction \(\mathcal{H}_\mathrm{hfs}\) given by equations
\(\mathcal{H}_Z=\frac{\mu_B}{\hbar}(g_J J_z+g_I I_z)B_z\)
and
\(\mathcal{H}_\mathrm{hfs}=A_\mathrm{hfs}I\cdot J + B_\mathrm{hfs}\frac{3(I\cdot J)^2+3/2 I\cdot J -I^2J^2}{2I(2I-1)2J(2J-1)}\).
- Parameters
n – principal,orbital, total orbital quantum numbers
l – principal,orbital, total orbital quantum numbers
j – principal,orbital, total orbital quantum numbers
B – Magnetic Field (units T)
- Returns
State energy \(E_z\) in SI units (Hz), state f, state mf
- Return type
- defectFittingRange = {}#
Used for AlkalineEarths to define minimum and maximum principal quantum number for which quantum defects are valid. Ranges are stored under keys defined as state terms ({‘stateLabel’:[minN, maxN]}, e.g. ‘1S0’). Dictionary returns array stating minimal and maximal principal quantum number for which quantum defects were fitted. For example:
limits = self.defectFittingRange['1S0'] print("Minimal n = %d" % limits[0]) print("Maximal n = %d" % limits[1]) 1
- energyLevelsExtrapolated = False#
flag that is turned to True if the energy levels of this atom were calculated by extrapolating with quantum defects values outside the quantum defect fitting range.
- getAverageInteratomicSpacing(temperature)#
Returns average interatomic spacing in atomic vapour
- getAverageSpeed(temperature)#
Average (mean) speed at a given temperature
- getBranchingRatio(jg, fg, mfg, je, fe, mfe, s=0.5)#
Branching ratio for decay from \(\vert j_e,f_e,m_{f_e} \rangle \rightarrow \vert j_g,f_g,m_{f_g}\rangle\)
\(b = \displaystyle\sum_q (2j_e+1)\left(\begin{matrix}f_1 & 1 & f_2 \\-m_{f1} & q & m_{f2}\end{matrix}\right)^2\vert \langle j_e,f_e\vert \vert er \vert\vert j_g,f_g\rangle\vert^2/|\langle j_e || er || j_g \rangle |^2\)
- Parameters
jg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
fg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
mfg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
je – total orbital, fine basis (total atomic) angular momentum,
fe – total orbital, fine basis (total atomic) angular momentum,
mfe – total orbital, fine basis (total atomic) angular momentum,
state (and projection of total angular momentum for excited) –
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
branching ratio
- Return type
- getC3term(n, l, j, n1, l1, j1, n2, l2, j2, s=0.5)#
C3 interaction term for the given two pair-states
- Calculates \(C_3\) intaraction term for
\(|n,l,j,n,l,j\rangle \leftrightarrow |n_1,l_1,j_1,n_2,l_2,j_2\rangle\)
- Parameters
n (int) – principal quantum number
l (int) – orbital angular momentum
j (float) – total angular momentum
n1 (int) – principal quantum number
l1 (int) – orbital angular momentum
j1 (float) – total angular momentum
n2 (int) – principal quantum number
l2 (int) – orbital angular momentum
j2 (float) – total angular momentum
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
\(C_3 = \frac{\langle n,l,j |er |n_1,l_1,j_1\rangle \langle n,l,j |er|n_2,l_2,j_2\rangle}{4\pi\varepsilon_0}\) (\(h\) Hz m \({}^3\)).
- Return type
- getC6term(n, l, j, n1, l1, j1, n2, l2, j2, s=0.5)#
C6 interaction term for the given two pair-states
Calculates \(C_6\) intaraction term for \(|n,l,j,n,l,j \rangle \leftrightarrow |n_1,l_1,j_1,n_2,l_2,j_2\rangle\). For details of calculation see Ref. 2.
- Parameters
n (int) – principal quantum number
l (int) – orbital angular momentum
j (float) – total angular momentum
n1 (int) – principal quantum number
l1 (int) – orbital angular momentum
j1 (float) – total angular momentum
n2 (int) – principal quantum number
l2 (int) – orbital angular momentum
j2 (float) – total angular momentum
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
\(C_6 = \frac{1}{4\pi\varepsilon_0} \frac{|\langle n,l,j |er|n_1,l_1,j_1\rangle|^2| \langle n,l,j |er|n_2,l_2,j_2\rangle|^2} {E(n_1,l_1,j_2,n_2,j_2,j_2)-E(n,l,j,n,l,j)}\) (\(h\) Hz m \({}^6\)).
- Return type
Example
We can reproduce values from Ref. 2 for C3 coupling to particular channels. Taking for example channels described by the Eq. (50a-c) we can get the values:
from arc import * channels = [[70,0,0.5, 70, 1,1.5, 69,1, 1.5],\ [70,0,0.5, 70, 1,1.5, 69,1, 0.5],\ [70,0,0.5, 69, 1,1.5, 70,1, 0.5],\ [70,0,0.5, 70, 1,0.5, 69,1, 0.5]] print(" = = = Caesium = = = ") atom = Caesium() for channel in channels: print("%.0f GHz (mu m)^6" % ( atom.getC6term(*channel) / C_h * 1.e27 )) print("\n = = = Rubidium = = =") atom = Rubidium() for channel in channels: print("%.0f GHz (mu m)^6" % ( atom.getC6term(*channel) / C_h * 1.e27 ))
Returns:
= = = Caesium = = = 722 GHz (mu m)^6 316 GHz (mu m)^6 383 GHz (mu m)^6 228 GHz (mu m)^6 = = = Rubidium = = = 799 GHz (mu m)^6 543 GHz (mu m)^6 589 GHz (mu m)^6 437 GHz (mu m)^6
which is in good agreement with the values cited in the Ref. 2. Small discrepancies for Caesium originate from slightly different quantum defects used in calculations.
References
- 2(1,2,3)
T. G. Walker, M. Saffman, PRA 77, 032723 (2008) https://doi.org/10.1103/PhysRevA.77.032723
- getDipoleMatrixElement(n1, l1, j1, mj1, n2, l2, j2, mj2, q, s=0.5)#
Dipole matrix element \(\langle n_1 l_1 j_1 m_{j_1} |e\mathbf{r}|\ n_2 l_2 j_2 m_{j_2}\rangle\) in units of \(a_0 e\)
- Parameters
l1 (n1.) – principal, orbital, total angular momentum, and projection of total angular momentum for state 1
j1 – principal, orbital, total angular momentum, and projection of total angular momentum for state 1
mj1 – principal, orbital, total angular momentum, and projection of total angular momentum for state 1
l2 (n2.) – principal, orbital, total angular momentum, and projection of total angular momentum for state 2
j2 – principal, orbital, total angular momentum, and projection of total angular momentum for state 2
mj2 – principal, orbital, total angular momentum, and projection of total angular momentum for state 2
q (int) – specifies transition that the driving field couples to, +1, 0 or -1 corresponding to driving \(\sigma^+\), \(\pi\) and \(\sigma^-\) transitions respectively.
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
dipole matrix element( \(a_0 e\))
- Return type
Example
For example, calculation of \(5 S_{1/2}m_j=-\frac{1}{2}\ \rightarrow 5 P_{3/2}m_j=-\frac{3}{2}\) transition dipole matrix element for laser driving \(\sigma^-\) transition:
from arc import * atom = Rubidium() # transition 5 S_{1/2} m_j=-0.5 -> 5 P_{3/2} m_j=-1.5 # for laser driving sigma- transition print(atom.getDipoleMatrixElement(5,0,0.5,-0.5,5,1,1.5,-1.5,-1))
- getDipoleMatrixElementHFS(n1, l1, j1, f1, mf1, n2, l2, j2, f2, mf2, q, s=0.5)#
Dipole matrix element for hyperfine structure resolved transitions \(\langle n_1 l_1 j_1 f_1 m_{f_1} |e\mathbf{r}|\ n_2 l_2 j_2 f_2 m_{f_2}\rangle\) in units of \(a_0 e\)
For hyperfine resolved transitions, the dipole matrix element is \(\langle n_1,\ell_1,j_1,f_1,m_{f1} | \ \mathbf{\hat{r}}\cdot \mathbf{\varepsilon}_q \ | n_2,\ell_2,j_2,f_2,m_{f2} \rangle = (-1)^{f_1-m_{f1}} \ \left( \ \begin{matrix} \ f_1 & 1 & f_2 \\ \ -m_{f1} & q & m_{f2} \ \end{matrix}\right) \ \langle n_1 \ell_1 j_1 f_1|| r || n_2 \ell_2 j_2 f_2 \rangle,\) where \(\langle n_1 \ell_1 j_1 f_1 ||r|| n_2 \ell_2 j_2 f_2 \rangle \ = (-1)^{j_1+I+f_2+1}\sqrt{(2f_1+1)(2f_2+1)} ~ \ \left\{ \begin{matrix}\ f_1 & 1 & f_2 \\ \ j_2 & I & j_1 \ \end{matrix}\right\}~ \ \langle n_1 \ell_1 j_1||r || n_2 \ell_2 j_2 \rangle.\)
- Parameters
n1 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
l1 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
j1 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
f1 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
mf1 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
n2 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 2
l2 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 2
j2 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 2
f2 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 2
mf2 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 2
q (int) – specifies transition that the driving field couples to, +1, 0 or -1 corresponding to driving \(\sigma^+\), \(\pi\) and \(\sigma^-\) transitions respectively.
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
dipole matrix element( \(a_0 e\))
- Return type
- getDipoleMatrixElementHFStoFS(n1, l1, j1, f1, mf1, n2, l2, j2, mj2, q, s=0.5)#
Dipole matrix element for transition from hyperfine resolved state to unresolved fine-structure state \(\langle n_1 l_1 j_1 f_1 m_{f_1} |e\mathbf{r}|\ n_2 l_2 j_2 m_{j_2}\rangle\) in units of \(a_0 e\)
For hyperfine resolved transitions, the dipole matrix element is \(\langle n_1,\ell_1,j_1,f_1,m_{f1} | \ \mathbf{\hat{r}}\cdot \mathbf{\varepsilon}_q \ | n_2,\ell_2,j_2,f_2,m_{f2} \rangle = (-1)^{f_1-m_{f1}} \ \left( \ \begin{matrix} \ f_1 & 1 & f_2 \\ \ -m_{f1} & q & m_{f2} \ \end{matrix}\right) \ \langle n_1 \ell_1 j_1 f_1|| r || n_2 \ell_2 j_2 f_2 \rangle,\) where \(\langle n_1 \ell_1 j_1 f_1 ||r|| n_2 \ell_2 j_2 f_2 \rangle \ = (-1)^{j_1+I+F_2+1}\sqrt{(2f_1+1)(2f_2+1)} ~ \ \left\{ \begin{matrix}\ F_1 & 1 & F_2 \\ \ j_2 & I & j_1 \ \end{matrix}\right\}~ \ \langle n_1 \ell_1 j_1||r || n_2 \ell_2 j_2 \rangle.\)
- Parameters
l1 (n1.) – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
j1 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
f1 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
mf1 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
l2 (n2.) – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total orbital angular momentum for state 2
j2 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total orbital angular momentum for state 2
mj2 – principal, orbital, total orbital, fine basis (total atomic) angular momentum, and projection of total orbital angular momentum for state 2
q (int) – specifies transition that the driving field couples to, +1, 0 or -1 corresponding to driving \(\sigma^+\), \(\pi\) and \(\sigma^-\) transitions respectively.
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
dipole matrix element( \(a_0 e\))
- Return type
- getEnergy(n, l, j, s=None)[source]#
Energy of the level relative to the ionisation level (in eV)
Returned energies are with respect to the center of gravity of the hyperfine-split states. If preferQuantumDefects =False (set during initialization) program will try use NIST energy value, if such exists, falling back to energy calculation with quantum defects if the measured value doesn’t exist. For preferQuantumDefects =True, program will calculate energies from quantum defects (useful for comparing quantum defect calculations with measured energy level values) if the principal quantum number of the requested state is larger than the minimal quantum principal quantum number self.minQuantumDefectN which sets minimal quantum number for which quantum defects still give good estimate of state energy (below this value saved energies will be used if existing).
- Parameters
- Returns
state energy (eV)
- Return type
- getEnergyDefect(n, l, j, n1, l1, j1, n2, l2, j2, s=0.5)#
Energy defect for the given two pair-states (one of the state has two atoms in the same state)
Energy difference between the states \(E(n_1,l_1,j_1,n_2,l_2,j_2) - E(n,l,j,n,l,j)\)
- Parameters
n (int) – principal quantum number
l (int) – orbital angular momentum
j (float) – total angular momentum
n1 (int) – principal quantum number
l1 (int) – orbital angular momentum
j1 (float) – total angular momentum
n2 (int) – principal quantum number
l2 (int) – orbital angular momentum
j2 (float) – total angular momentum
s (float) – optional. Spin angular momentum (default 0.5 for Alkali)
- Returns
energy defect (SI units: J)
- Return type
- getEnergyDefect2(n, l, j, nn, ll, jj, n1, l1, j1, n2, l2, j2, s=0.5)#
Energy defect for the given two pair-states
Energy difference between the states \(E(n_1,l_1,j_1,n_2,l_2,j_2) - E(n,l,j,nn,ll,jj)\)
See pair-state energy defects example snippet.
- Parameters
n (int) – principal quantum number
l (int) – orbital angular momentum
j (float) – total angular momentum
nn (int) – principal quantum number
ll (int) – orbital angular momentum
jj (float) – total angular momentum
n1 (int) – principal quantum number
l1 (int) – orbital angular momentum
j1 (float) – total angular momentum
n2 (int) – principal quantum number
l2 (int) – orbital angular momentum
j2 (float) – total angular momentum
s (float) – optional. Spin angular momentum (default 0.5 for Alkali)
- Returns
energy defect (SI units: J)
- Return type
- getHFSCoefficients(n, l, j, s=None)#
Returns hyperfine splitting coefficients for state \(n\), \(l\), \(j\).
- getHFSEnergyShift(j, f, A, B=0, s=0.5)#
Energy shift of HFS from centre of mass \(\Delta E_\mathrm{hfs}\)
\(\Delta E_\mathrm{hfs} = \frac{A}{2}K+B\frac{\frac{3}{2}K(K+1)-2I(I+1)J(J+1)}{2I(2I-1)2J(2J-1)}\)
where \(K=F(F+1)-I(I+1)-J(J+1)\)
- Parameters
- Returns
Energy shift ( \(\Delta E_\mathrm{hfs}\))
- Return type
- getLandegf(l, j, f, s=0.5)#
Lande g-factor \(g_F\simeq g_J\frac{f(f+1)-I(I+1)+j(j+1)}{2f(f+1)}\)
- getLandegfExact(l, j, f, s=0.5)#
Lande g-factor \(g_F\) \(g_F=g_J\frac{f(f+1)-I(I+1)+j(j+1)}{2f(f+1)}+g_I\frac{f(f+1)+I(I+1)-j(j+1)}{2f(f+1)}\)
- getLandegj(l, j, s=0.5)#
Lande g-factor \(g_J\simeq 1+\frac{j(j+1)+s(s+1)-l(l+1)}{2j(j+1)}\)
- getLandegjExact(l, j, s=0.5)#
Lande g-factor \(g_J=g_L\frac{j(j+1)-s(s+1)+l(l+1)}{2j(j+1)}+g_S\frac{j(j+1)+s(s+1)-l(l+1)}{2j(j+1)}\)
- getLiteratureDME(n1, l1, j1, n2, l2, j2, s=0)[source]#
Returns literature information on requested transition.
- Parameters
n1 – one of the states we are coupling
l1 – one of the states we are coupling
j1 – one of the states we are coupling
n2 – the other state to which we are coupling
l2 – the other state to which we are coupling
j2 – the other state to which we are coupling
s – (optional) spin of the state. Default s=0.
- Returns
hasLiteratureValue?, dme, referenceInformation
If Boolean value is True, a literature value for dipole matrix element was found and reduced DME in J basis is returned as the number. The third returned argument (array) contains additional information about the literature value in the following order [ typeOfSource, errorEstimate , comment , reference, reference DOI] upon success to find a literature value for dipole matrix element:
typeOfSource=1 if the value is theoretical calculation; otherwise, if it is experimentally obtained value typeOfSource=0
comment details where within the publication the value can be found
errorEstimate is absolute error estimate
reference is human-readable formatted reference
reference DOI provides link to the publication.
Boolean value is False, followed by zero and an empty array if no literature value for dipole matrix element is found.
- Return type
Note
The literature values are stored in /data folder in <element name>_literature_dme.csv files as a ; separated values. Each row in the file consists of one literature entry, that has information in the following order:
n1
l1
j1
n2
l2
j2
s
dipole matrix element reduced l basis (a.u.)
comment (e.g. where in the paper value appears?)
value origin: 1 for theoretical; 0 for experimental values
accuracy
source (human readable formatted citation)
doi number (e.g. 10.1103/RevModPhys.82.2313 )
If there are several values for a given transition, program outputs the value that has smallest error (under column accuracy). The list of values can be expanded - every time program runs this file is read and the list is parsed again for use in calculations.
- getMagneticDipoleMatrixElementHFS(l, j, f1, mf1, f2, mf2, q, s=0.5)#
Magnetic dipole matrix element \(\langle f_1,m_{f_1} \vert \mu_q \vert f_2,m_{f_2}\rangle\) for transitions from \(\vert f_1,m_{f_1}\rangle\rightarrow\vert f_2,m_{f_2}\rangle\) within the same \(n,\ell,j\) state in units of \(\mu_B B_q\).
The magnetic dipole matrix element is given by \(\langle f_1,m_{f_1}\vert \mu_q \vert f_2,m_{f_2}\rangle = g_J \mu_B B_q (-1)^{f_2+j+I+1+f_1-m_{f_1}} \sqrt{(2f_1+1)(2f_2+1)j(j+1)(2j+1)} \begin{pmatrix}f_1&1&f_2\\-m_{f_1} & -q & m_{f_2}\end{pmatrix} \begin{Bmatrix}f_1&1&f_2\\j & I & j\end{Bmatrix}\)
- Args:
- l, j, f1, mf1: orbital, total orbital,
fine basis (total atomic) angular momentum,total anuglar momentum and projection of total angular momentum for state 1
- f2,mf2: principal, orbital, total orbital,
fine basis (total atomic) angular momentum, and projection of total orbital angular momentum for state 2
- q (int): specifies transition that the driving field couples to,
+1, 0 or -1 corresponding to driving \(\sigma^+\), \(\pi\) and \(\sigma^-\) transitions respectively.
- s (float): optional, total spin angular momentum of state.
By default 0.5 for Alkali atoms.
- Returns:
float: magnetic dipole matrix element (in units of \(\mu_BB_q\))
- getNumberDensity(temperature)#
Atom number density at given temperature
- getPressure(temperature)#
Vapour pressure (in Pa) at given temperature
- getQuadrupoleMatrixElement(n1, l1, j1, n2, l2, j2, s=0.5)[source]#
Radial part of the quadrupole matrix element
Calculates \(\int \mathbf{d}r~R_{n_1,l_1,j_1}(r)\cdot R_{n_1,l_1,j_1}(r) \cdot r^4\). See Quadrupole calculation example snippet .
- Parameters
n1 (int) – principal quantum number of state 1
l1 (int) – orbital angular momentum of state 1
j1 (float) – total angular momentum of state 1
n2 (int) – principal quantum number of state 2
l2 (int) – orbital angular momentum of state 2
j2 (float) – total angular momentum of state 2
s (float) – optional. Spin of the state. Default 0.5 is for Alkali
- Returns
quadrupole matrix element (\(a_0^2 e\)).
- Return type
- getQuantumDefect(n, l, j, s=0.5)#
Quantum defect of the level.
For an example, see Rydberg energy levels example snippet.
- Parameters
- Returns
quantum defect
- Return type
- getRabiFrequency(n1, l1, j1, mj1, n2, l2, j2, q, laserPower, laserWaist, s=0.5)#
Returns a Rabi frequency for resonantly driven atom in a center of TEM00 mode of a driving field
- Parameters
n1 – state from which we are driving transition
l1 – state from which we are driving transition
j1 – state from which we are driving transition
mj1 – state from which we are driving transition
n2 – state to which we are driving transition
l2 – state to which we are driving transition
j2 – state to which we are driving transition
q – laser polarization (-1,0,1 correspond to \(\sigma^-\), \(\pi\) and \(\sigma^+\) respectively)
laserPower – laser power in units of W
laserWaist – laser \(1/e^2\) waist (radius) in units of m
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
Frequency in rad \(^{-1}\). If you want frequency in Hz, divide by returned value by \(2\pi\)
- Return type
- getRabiFrequency2(n1, l1, j1, mj1, n2, l2, j2, q, electricFieldAmplitude, s=0.5)#
Returns a Rabi frequency for resonant excitation with a given electric field amplitude
- Parameters
n1 – state from which we are driving transition
l1 – state from which we are driving transition
j1 – state from which we are driving transition
mj1 – state from which we are driving transition
n2 – state to which we are driving transition
l2 – state to which we are driving transition
j2 – state to which we are driving transition
q – laser polarization (-1,0,1 correspond to \(\sigma^-\), \(\pi\) and \(\sigma^+\) respectively)
electricFieldAmplitude – amplitude of electric field driving (V/m)
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
Frequency in rad \(^{-1}\). If you want frequency in Hz, divide by returned value by \(2\pi\)
- Return type
- getRadialCoupling(n, l, j, n1, l1, j1, s=0.5)#
Returns radial part of the coupling between two states (dipole and quadrupole interactions only)
- Parameters
n1 (int) – principal quantum number
l1 (int) – orbital angular momentum
j1 (float) – total angular momentum
n2 (int) – principal quantum number
l2 (int) – orbital angular momentum
j2 (float) – total angular momentum
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
radial coupling strength (in a.u.), or zero for forbidden transitions in dipole and quadrupole approximation.
- Return type
- getRadialMatrixElement(n1, l1, j1, n2, l2, j2, s=None, useLiterature=True)[source]#
Radial part of the dipole matrix element
Calculates \(\int \mathbf{d}r~R_{n_1,l_1,j_1}(r)\cdot R_{n_1,l_1,j_1}(r) \cdot r^3\).
- Parameters
n1 (int) – principal quantum number of state 1
l1 (int) – orbital angular momentum of state 1
j1 (float) – total angular momentum of state 1
n2 (int) – principal quantum number of state 2
l2 (int) – orbital angular momentum of state 2
j2 (float) – total angular momentum of state 2
s (float) – is required argument, total spin angular momentum of state. Specify s=0 for singlet state or s=1 for triplet state.
useLiterature (bool) – optional, should literature values for dipole matrix element be used if existing? If true, compiled values stored in literatureDMEfilename variable for a given atom (file is stored locally at ~/.arc-data/), will be checked, and if the value is found, selects the value with smallest error estimate (if there are multiple entries). If no value is found, it will default to numerical integration of wavefunctions. By default True.
- Returns
dipole matrix element (\(a_0 e\)).
- Return type
- getReducedMatrixElementJ(n1, l1, j1, n2, l2, j2, s=0.5)#
Reduced matrix element in \(J\) basis (symmetric notation)
- Parameters
n1 (int) – principal quantum number of state 1
l1 (int) – orbital angular momentum of state 1
j1 (float) – total angular momentum of state 1
n2 (int) – principal quantum number of state 2
l2 (int) – orbital angular momentum of state 2
j2 (float) – total angular momentum of state 2
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
reduced dipole matrix element in \(J\) basis \(\langle j || er || j' \rangle\) (\(a_0 e\)).
- Return type
- getReducedMatrixElementJ_asymmetric(n1, l1, j1, n2, l2, j2, s=0.5)#
Reduced matrix element in \(J\) basis, defined in asymmetric notation.
Note that notation for symmetric and asymmetricly defined reduced matrix element is not consistent in the literature. For example, notation is used e.g. in Steck 1 is precisely the oposite.
Note
Note that this notation is asymmetric: \(( j||e r ||j' ) \neq ( j'||e r ||j )\). Relation between the two notation is \(\langle j||er||j' \rangle=\sqrt{2j+1} ( j ||er ||j')\). This function always returns value for transition from lower to higher energy state, independent of the order of states entered in the function call.
- Parameters
n1 (int) – principal quantum number of state 1
l1 (int) – orbital angular momentum of state 1
j1 (float) – total angular momentum of state 1
n2 (int) – principal quantum number of state 2
l2 (int) – orbital angular momentum of state 2
j2 (float) – total angular momentum of state 2
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
reduced dipole matrix element in Steck notation \(( j || er || j' )\) (\(a_0 e\)).
- Return type
- 1
Daniel A. Steck, “Cesium D Line Data,” (revision 2.0.1, 2 May 2008). http://steck.us/alkalidata
- getReducedMatrixElementL(n1, l1, j1, n2, l2, j2, s=0.5)#
Reduced matrix element in \(L\) basis (symmetric notation)
- Parameters
- Returns
reduced dipole matrix element in \(L\) basis \(\langle l || er || l' \rangle\) (\(a_0 e\)).
- Return type
- getSaturationIntensity(ng, lg, jg, fg, mfg, ne, le, je, fe, mfe, s=0.5)#
Saturation Intensity \(I_\mathrm{sat}\) for transition \(\vert j_g,f_g,m_{f_g}\rangle\rightarrow\vert j_e,f_e,m_{f_e}\rangle\) in units of \(\mathrm{W}/\mathrm{m}^2\).
\(I_\mathrm{sat} = \frac{c\epsilon_0\Gamma^2\hbar^2}{4\vert \epsilon_q\cdot\mathrm{d}\vert^2}\)
- Parameters
ng – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
lg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
jg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
fg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
mfg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
ne – total orbital, fine basis (total atomic) angular momentum,
le – total orbital, fine basis (total atomic) angular momentum,
je – total orbital, fine basis (total atomic) angular momentum,
fe – total orbital, fine basis (total atomic) angular momentum,
mfe – total orbital, fine basis (total atomic) angular momentum,
state (and projection of total angular momentum for excited) –
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
Saturation Intensity in units of \(\mathrm{W}/\mathrm{m}^2\)
- Return type
- getSaturationIntensityIsotropic(ng, lg, jg, fg, ne, le, je, fe)#
Isotropic Saturation Intensity \(I_\mathrm{sat}\) for transition \(f_g\rightarrow f_e\) averaged over all polarisations in units of \(\mathrm{W}/\mathrm{m}^2\).
\(I_\mathrm{sat} = \frac{c\epsilon_0\Gamma^2\hbar^2}{4\vert \epsilon_q\cdot\mathrm{d}\vert^2}\)
- Parameters
ng – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
lg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
jg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
fg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
mfg – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for ground state
ne – total orbital, fine basis (total atomic) angular momentum,
le – total orbital, fine basis (total atomic) angular momentum,
je – total orbital, fine basis (total atomic) angular momentum,
fe – total orbital, fine basis (total atomic) angular momentum,
mfe – total orbital, fine basis (total atomic) angular momentum,
state (and projection of total angular momentum for excited) –
- Returns
Saturation Intensity in units of \(\mathrm{W}/\mathrm{m}^2\)
- Return type
- getSphericalMatrixElementHFStoFS(j1, f1, mf1, j2, mj2, q)#
Spherical matrix element for transition from hyperfine resolved state to unresolved fine-structure state \(\langle f,m_f \vert\mu_q\vert j',m_j'\rangle\) in units of \(\langle j\vert\vert\mu\vert\vert j'\rangle\)
- Parameters
j1 – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
f1 – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
mf1 – total orbital, fine basis (total atomic) angular momentum, and projection of total angular momentum for state 1
j2 – total orbital, fine basis (total atomic) angular momentum, and projection of total orbital angular momentum for state 2
mj2 – total orbital, fine basis (total atomic) angular momentum, and projection of total orbital angular momentum for state 2
q (int) – specifies transition that the driving field couples to, +1, 0 or -1 corresponding to driving \(\sigma^+\), \(\pi\) and \(\sigma^-\) transitions respectively.
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
spherical dipole matrix element( \(\langle j\vert\vert\mu\vert\vert j'\rangle\))
- Return type
- getStateLifetime(n, l, j, temperature=0, includeLevelsUpTo=0, s=0)[source]#
Returns the lifetime of the state (in s)
For non-zero temperatures, user must specify up to which principal quantum number levels, that is above the initial state, should be included in order to account for black-body induced transitions to higher lying states. See Rydberg lifetimes example snippet.
- Parameters
n (int,int,float) – specifies state whose lifetime we are calculating
l (int,int,float) – specifies state whose lifetime we are calculating
j (int,int,float) – specifies state whose lifetime we are calculating
temperature – optional. Temperature at which the atom environment is, measured in K. If this parameter is non-zero, user has to specify transitions up to which state (due to black-body decay) should be included in calculation.
includeLevelsUpTo (int) – optional and not needed for atom lifetimes calculated at zero temperature. At non zero temperatures, this specify maximum principal quantum number of the state to which black-body induced transitions will be included. Minimal value of the parameter in that case is \(n+1\)
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
State lifetime in units of s (seconds)
- Return type
See also
getTransitionRatefor calculating rates of individual transition rates between the two states
- getTransitionFrequency(n1, l1, j1, n2, l2, j2, s=0.5, s2=None)#
Calculated transition frequency in Hz
Returned values is given relative to the centre of gravity of the hyperfine-split states.
- Parameters
n1 (int) – principal quantum number of the state from which we are going
l1 (int) – orbital angular momentum of the state from which we are going
j1 (float) – total angular momentum of the state from which we are going
n2 (int) – principal quantum number of the state to which we are going
l2 (int) – orbital angular momentum of the state to which we are going
j2 (float) – total angular momentum of the state to which we are going
s (float) – optional, spin of the intial state (for Alkali this is fixed to 0.5)
s2 (float) – optional, spin of the final state If not set, defaults to the same value as
s
- Returns
transition frequency (in Hz). If the returned value is negative, level from which we are going is above the level to which we are going.
- Return type
- getTransitionRate(n1, l1, j1, n2, l2, j2, temperature=0.0, s=0.5)#
Transition rate due to coupling to vacuum modes (black body included)
Calculates transition rate from the first given state to the second given state \(|n_1,l_1,j_1\rangle \rightarrow |n_2,j_2,j_2\rangle\) at given temperature due to interaction with the vacuum field. For zero temperature this returns Einstein A coefficient. For details of calculation see Ref. 3 and Ref. 4. See Black-body induced population transfer example snippet.
- Parameters
n1 (int) – principal quantum number
l1 (int) – orbital angular momentum
j1 (float) – total angular momentum
n2 (int) – principal quantum number
l2 (int) – orbital angular momentum
j2 (float) – total angular momentum
[temperature] (float) – temperature in K
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
transition rate in s \({}^{-1}\) (SI)
- Return type
References
- 3
C. E. Theodosiou, PRA 30, 2881 (1984) https://doi.org/10.1103/PhysRevA.30.2881
- 4
I. I. Beterov, I. I. Ryabtsev, D. B. Tretyakov, and V. M. Entin, PRA 79, 052504 (2009) https://doi.org/10.1103/PhysRevA.79.052504
- getTransitionWavelength(n1, l1, j1, n2, l2, j2, s=0.5, s2=None)#
Calculated transition wavelength (in vacuum) in m.
Returned values is given relative to the centre of gravity of the hyperfine-split states.
- Parameters
n1 (int) – principal quantum number of the state from which we are going
l1 (int) – orbital angular momentum of the state from which we are going
j1 (float) – total angular momentum of the state from which we are going
n2 (int) – principal quantum number of the state to which we are going
l2 (int) – orbital angular momentum of the state to which we are going
j2 (float) – total angular momentum of the state to which we are going
s (float) – optional, spin of the intial state (for Alkali this is fixed to 0.5)
s2 (float) – optional, spin of the final state. If not set, defaults to same value as
s
- Returns
transition wavelength (in m). If the returned value is negative, level from which we are going is above the level to which we are going.
- Return type
- getZeemanEnergyShift(l, j, mj, magneticFieldBz, s=0.5)#
Retuns linear (paramagnetic) Zeeman shift.
\(\mathcal{H}_P=\frac{\mu_B B_z}{\hbar}(\hat{L}_{\rm z}+\ g_{\rm S}S_{\rm z})\)
- Parameters
l (int) – orbital angular momentum
j (float) – total angular momentum
mj (float) – projection of total angular momentum alon z-axis
magneticFieldBz (float) – applied magnetic field (alon z-axis only) in units of T (Tesla)
s (float) – optional, total spin angular momentum of state. By default 0.5 for Alkali atoms.
- Returns
energy offset of the state (in J)
- Return type
- groundStateRamanTransition(Pa, wa, qa, Pb, wb, qb, Delta, f0, mf0, f1, mf1, ne, le, je)#
Returns two-photon Rabi frequency \(\Omega_R\), differential AC Stark shift \(\Delta_\mathrm{AC}\) and probability to scatter a photon during a \(\pi\)-pulse \(P_\mathrm{sc}\) for two-photon ground-state Raman transitions from \(\vert f_g,m_{f_g}\rangle\rightarrow\vert nL_{j_r} j_r,m_{j_r}\rangle\) via an intermediate excited state \(n_e,\ell_e,j_e\).
\(\Omega_R=\displaystyle\sum_{f_e,m_{f_e}}\frac{\Omega^a_{0\rightarrow f_e}\Omega^b_{1\rightarrow f_e}}{2(\Delta-\Delta_{f_e})},\)
\(\Delta_{\mathrm{AC}} = \displaystyle\sum_{f_e,m_{f_e}}\left[\frac{\vert\Omega^a_{0\rightarrow f_e}\vert^2-\vert\Omega^b_{1\rightarrow f_e}\vert^2}{4(\Delta-\Delta_{f_e})}+\frac{\vert\Omega^a_{1\rightarrow f_e}\vert^2}{4(\Delta+\omega_{01}-\Delta_{f_e})}-\frac{\vert\Omega^b_{0\rightarrow f_e}\vert^2}{4(\Delta-\omega_{01}-\Delta_{f_e})}\right],\)
\(P_\mathrm{sc} =\frac{\Gamma_e t_\pi}{2}\displaystyle\sum_{f_e,m_{f_e}}\left[\frac{\vert\Omega^a_{0\rightarrow f_e}\vert^2}{2(\Delta-\Delta_{f_e})^2}+\frac{\vert\Omega^b_{1\rightarrow f_e}\vert^2}{2(\Delta-\Delta_{f_e})^2}+\frac{\vert\Omega^a_{1\rightarrow f_e}\vert^2}{4(\Delta+\omega_{01}-\Delta_{f_e})^2}+\frac{\vert\Omega^b_{0\rightarrow f_e}\vert^2}{4(\Delta-\omega_{01}-\Delta_{f_e})^2}\right]\)
where \(\tau_\pi=\pi/\Omega_R\).
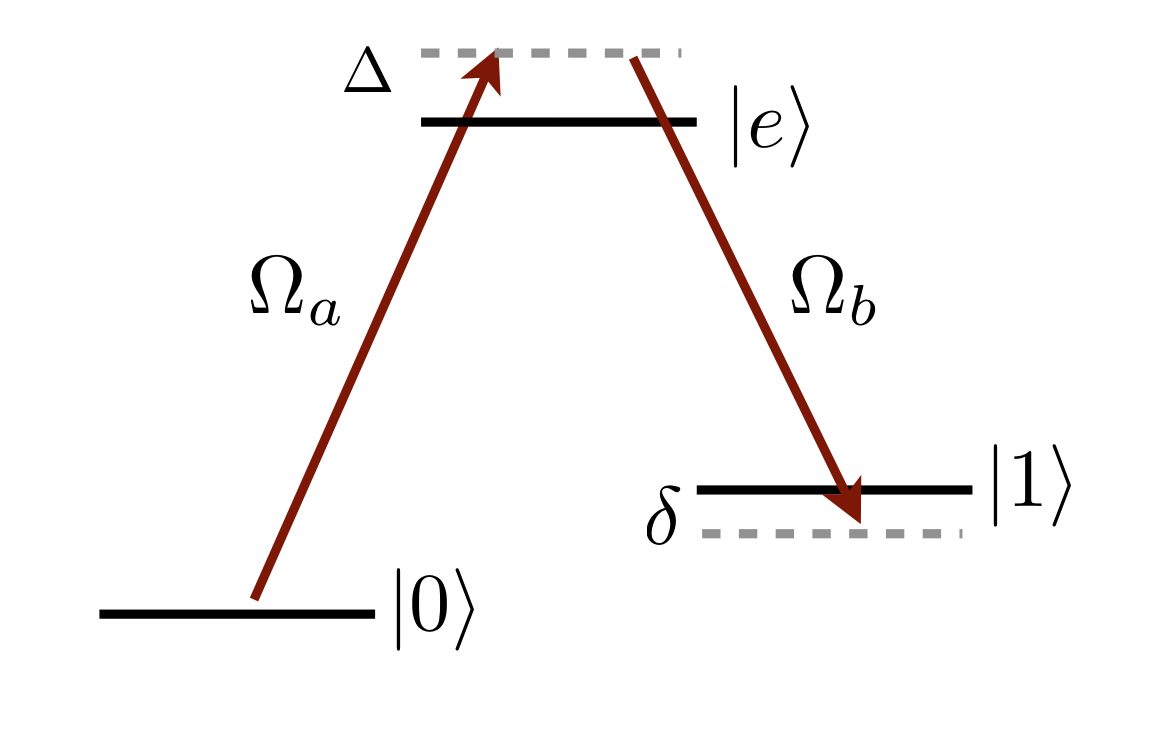
- Parameters
Pa – power (W), of laser a \(\vert 0 \rangle\rightarrow\vert e\rangle\)
wa – beam waist (m) of laser a \(\vert 0 \rangle\rightarrow\vert e\rangle\)
qa – polarisation (+1, 0 or -1 corresponding to driving \(\sigma^+\), \(\pi\) and \(\sigma^-\)) of laser a \(\vert 0 \rangle\rightarrow\vert e\rangle\)
Pb – power (W) of laser b \(\vert 1 \rangle\rightarrow\vert e\rangle\)
wb – beam waist (m) of laser b \(\vert 1 \rangle\rightarrow\vert e\rangle\)
qb – polarisation (+1, 0 or -1 corresponding to driving \(\sigma^+\), \(\pi\) and \(\sigma^-\)) of laser b \(\vert 1 \rangle\rightarrow\vert e\rangle\)
Delta – Detuning from excited state centre of mass (rad \(\mathrm{s}^{-1}\))
f0 – Lower hyperfine level
mf0 – Lower hyperfine level
f1 – Upper hyperfine level
mf1 – Upper hyperfine level
ne – principal, orbital, total orbital quantum numbers of excited state
le – principal, orbital, total orbital quantum numbers of excited state
je – principal, orbital, total orbital quantum numbers of excited state
- Returns
Two-Photon Rabi frequency \(\Omega_R\) (units \(\mathrm{rads}^{-1}\)), differential AC Stark shift \(\Delta_\mathrm{AC}\) (units \(\mathrm{rads}^{-1}\)) and probability to scatter a photon during a \(\pi\)-pulse \(P_\mathrm{sc}\)
- Return type
- levelDataFromNIST = ''#
file with .csv data, each row is [n, l, s, j, energy, source, absolute uncertanty]
- minQuantumDefectN = None#
Not used with DivalentAtom, see
defectFittingRangeinstead.
- modelPotential_coef = {}#
Model potential parameters fitted from experimental observations for different l (electron angular momentum)
- quantumDefect = [[[0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0]], [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0]], [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0]], [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0], [0.0, 0.0, 0.0, 0.0, 0.0, 0.0]]]#
Contains list of modified Rydberg-Ritz coefficients for calculating quantum defects for [[ \(^1S_{0},^1P_{1},^1D_{2},^1F_{3}\)], [ \(^3S_{0},^3P_{0},^3D_{1},^3F_{2}\)], [ \(^3S_{0},^3P_{1},^3D_{2},^3F_{3}\)], [ \(^3S_{1},^3P_{2},^3D_{3},^3F_{4}\)]].
- radialWavefunction(l, s, j, stateEnergy, innerLimit, outerLimit, step)[source]#
Not implemented for Alkaline earths
- twoPhotonRydbergExcitation(Pp, wp, qp, Pc, wc, qc, Delta, fg, mfg, ne, le, je, nr, lr, jr, mjr)#
Returns two-photon Rabi frequency \(\Omega_R\), ground AC Stark shift \(\Delta_{\mathrm{AC}_g}\), Rydberg state AC Stark shift \(\Delta_{\mathrm{AC}_r}\) and probability to scatter a photon during a \(\pi\)-pulse \(P_\mathrm{sc}\) for two-photon excitation from \(\vert f_h,m_{f_g}\rangle\rightarrow \vert j_r,m_{j_r}\rangle\) via intermediate excited state
\(\Omega_R=\displaystyle\sum_{f_e,m_{f_e}}\frac{\Omega_p^{g\rightarrow f_e}\Omega_c^{f_e\rightarrow r}}{2(\Delta-\Delta_{f_e})}\)
\(\Delta_{\mathrm{AC}_g} = \displaystyle\sum_{f_e,m_{f_e}}\frac{\vert\Omega_p^{g\rightarrow f_e}\vert^2}{4(\Delta-\Delta_{f_e})}\)
\(\Delta_{\mathrm{AC}_r} = \displaystyle\sum_{f_e,m_{f_e}}\frac{\vert\Omega_p^{g\rightarrow f_e}\vert^2}{4(\Delta-\Delta_{f_e})}\)
\(P_\mathrm{sc} = \frac{\Gamma_et_\pi}{2}\displaystyle\sum_{f_e,m_{f_e}}\left[\frac{\vert\Omega_p^{g\rightarrow f_e}\vert^2}{2(\Delta-\Delta_{f_e})^2}+\frac{\vert\Omega_c^{f_e\rightarrow r}\vert^2}{2(\Delta-\Delta_{f_e})^2}\right]\)
where \(\tau_\pi=\pi/\Omega_R\).
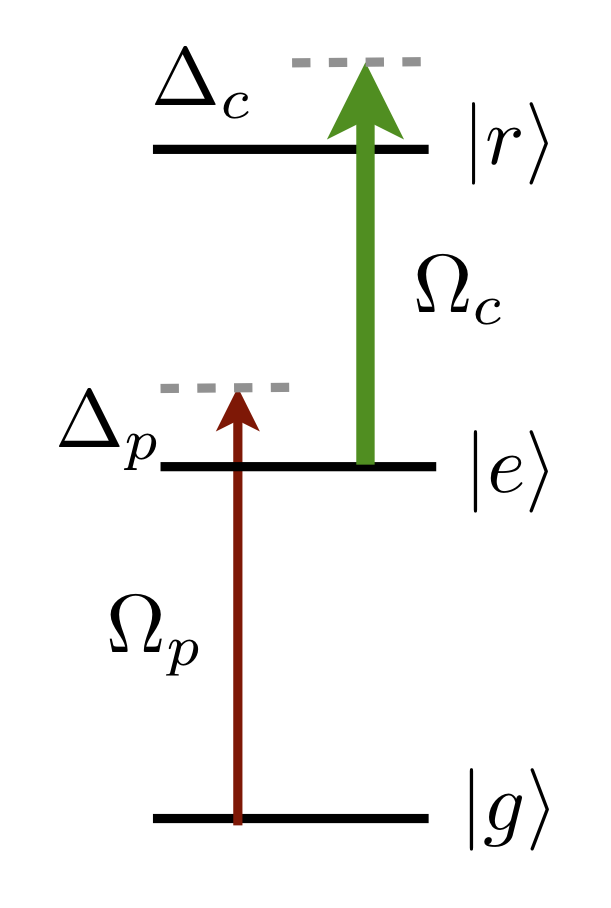
- Parameters
Pp – power (W) of probe laser \(\vert g \rangle\rightarrow\vert e\rangle\)
wp – beam waist (m) of probe laser \(\vert g \rangle\rightarrow\vert e\rangle\)
qp – polarisation (+1, 0 or -1 corresponding to driving \(\sigma^+\),:math:pi and \(\sigma^-\)) of probe laser \(\vert g \rangle\rightarrow\vert e\rangle\)
Pb – power (W) of coupling laser \(\vert e\rangle\rightarrow\vert r\rangle\)
wb – beam waist (m) of coupling laser \(\vert e\rangle\rightarrow\vert r\rangle\)
qb – polarisation (+1, 0 or -1 corresponding to driving \(\sigma^+\),:math:pi and \(\sigma^-\)) of coupling laser \(\vert e\rangle\rightarrow\vert r\rangle\)
Delta – Detuning from excited state centre of mass (rad s:math:^{-1})
fg – ground state hyperfine state
mfg – projection of ground state hyperfine state
f1 – upper hyperfine state
mf1 – upper hyperfine state
ne – principal quantum numbers of excited state
le – orbital angular momentum of excited state
je – total angular momentum of excited state
nr – principal quantum number of target Rydberg state
lr – orbital angular momentum of target Rydberg state
jr – total angular momentum of target Rydberg state
mjr – projection of total angular momenutm of target Rydberg state
- Returns
Two-Photon Rabi frequency \(\Omega_R\) (units \(\mathrm{rads}^{-1}\)), ground-state AC Stark shift \(\Delta_{\mathrm{AC}_g}\) (units \(\mathrm{rads}^{-1}\)) Rydberg-state AC Stark shift \(\Delta_{\mathrm{AC}_r}\) (units \(\mathrm{rads}^{-1}\)) and probability to scatter a photon during a \(\pi\)-pulse \(P_\mathrm{sc}\)
- Return type
- updateDipoleMatrixElementsFile()#
Updates the file with pre-calculated dipole matrix elements.
This function will add the the file all the elements that have been calculated in the previous run, allowing quick access to them in the future calculations.